Exploring Quantum Chemistry
The pKa of an acid is a measure of its strength, which is a crucial factor in its reactivity. Accurately predicting pKa values allows us to understand the relative reactivity of different acids, which can be important in various chemical processes, such as synthesis, catalysis, and biochemistry. It can also guide the development of new materials with desired properties. Let’s see how well quantum chemistry performs in evaluating this essential property by examining the results of a joint study by researchers from the Ruhr University Bochum and BIOVIA [1].
The solvent under investigation is acetone. Acetone is a versatile material with a wide range of applications. In everyday life, it is maybe best known for its cleaning abilities as nail polish remover. Yet, it is also an essential solvent in various industries. Acetone is used as a solvent for extracting compounds from samples for analysis, to dissolve resins and coatings or active pharmaceutical ingredients (APIs) and other components of pharmaceutical formulations, and to disinfect and clean instruments. It is a common solvent in organic chemistry, enabling a wide range of reactions.
Computational methodology of choice is COSMO-RS, also known as Conductor-Like Screening Model for Real Solvents. It is a powerful computational tool with widespread applications across various industries. Its ability to predict and interpret various physicochemical properties of compounds in different solvents makes it a valuable asset in many industries, e.g. chemical engineering, materials science, pharmaceuticals, agrochemistry, and flavor and fragrance industries. COSMO-RS can be applied to design new products, optimize processes, and ensure compliance with environmental regulations.
Exploring the Link Between pKa and Quantum Predictions for Reaction Insights
A linear free-energy relationship (LFER) can be applied between the pKa value and the change of the Gibbs free energy of dissociation (ΔGdiss). This is because the pKa value is a measure of the equilibrium constant for the dissociation reaction, and the Gibbs free energy change is another way of expressing the equilibrium constant.
The Gibbs free energy change of dissociation (ΔGdiss) can be predicted with quantum chemical methods, such as density functional theory (DFT) and coupled cluster theory (CC). These methods provide a detailed description of the electronic structure and energies of molecules, which is essential for accurate ΔGdiss predictions.
Quantum chemical methods can be used to calculate the Gibbs free energy change of dissociation by simulating the dissociation reaction in a quantum mechanical environment. This involves calculating the energy of the reactants, the products, and the transition state. The Gibbs free energy change is then calculated from the difference in energy between the reactants and the products.
Density functional theory (DFT) is a widely used and computationally efficient method for predicting molecular properties. DFT has been successfully used to predict the ΔGdiss of a variety of molecules. The accuracy of ΔGdiss predictions using quantum chemical methods can be improved by using higher-level theory, such as MP2 or CCSD(T). However, these methods are more computationally expensive, which can limit their practical application to small molecules. In general, DFT is a good choice for predicting ΔGdiss for most organic acids and bases.
Accomplishments and Milestones
Scientists from the Theoretical Chemistry Department of the Ruhr University Bochum and BIOVIA determined the LFER parameters for usage with COSMO-RS theory [1].
A set of 120 acids in the solvent acetone for which experimental reference data is available has been collected. The theoretical prediction of ΔGdiss values has been performed with BIOVIA TURBOMOLE [2], COSMOconf [3] and COSMOtherm [4].
For the organic acids considered in the work LFER fits yield very good linear correlations between ΔGdiss computed with COSMO-RS and DFT and the experimental pKa values within each compound class.
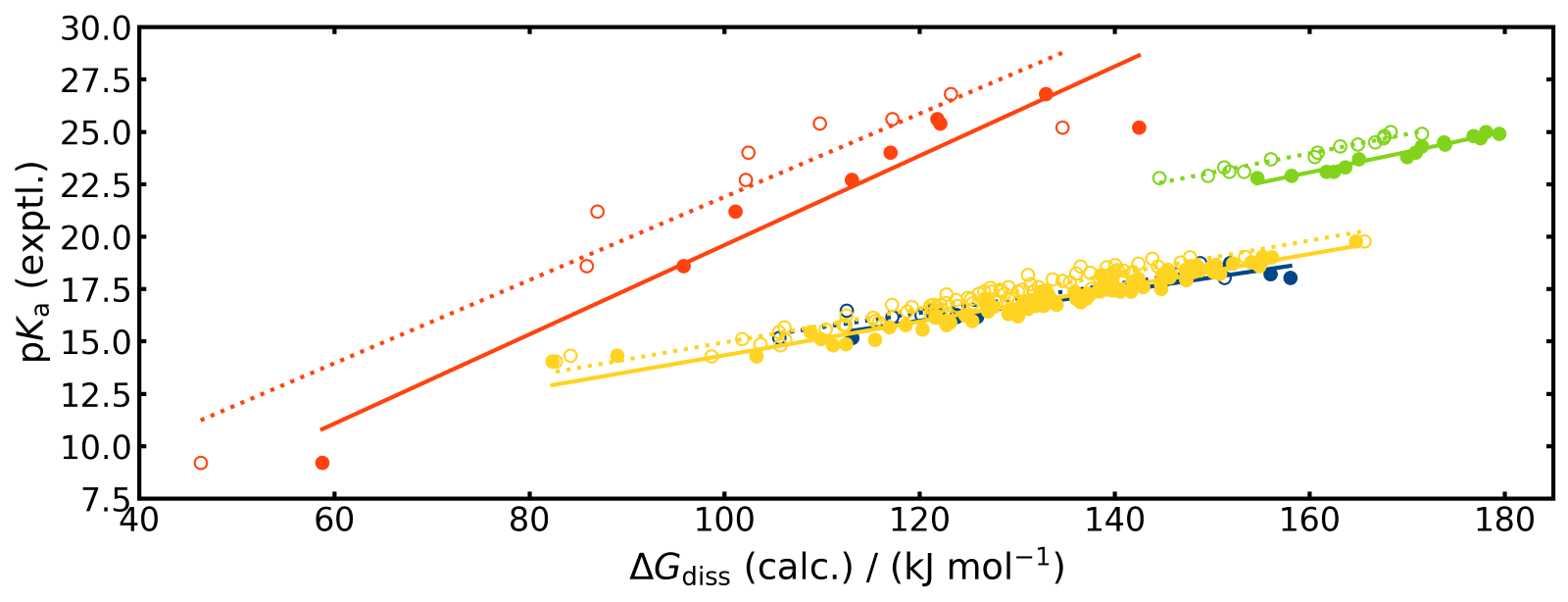
Figure 1: The linear relationship between pKa and ΔGdiss plotted.
Conclusion
The accurate prediction of pKa values makes life for researchers easier in many industrial fields. The application of such prediction has now been expanded to acids in the solvent acetone.
The prediction of acid pKa values in acetone using COSMO-RS is an important tool for understanding chemical reactivity, designing pharmaceuticals, developing new materials, conducting biochemical studies, and conducting efficient computational analysis. The workflow established in this research has significant promise as a replacement for non-trivial and relatively costly experimental measurements of extremely low acid dissociation constants in organic solvents.
References
- N. Sülzner, J. Haberhauer, C. Hättig, A. Hellweg, J. Comput. Chem. 2022, 43(15), 1011; https://doi.org/10.1002/jcc.26864
- TURBOMOLE V7.3, A development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH 2007, 2018; http://www.turbomole.com.
- BIOVIA COSMOconf, Release 2020; Dassault Systèmes. https://www.3ds.com/fileadmin/PRODUCTS-SERVICES/BIOVIA/PDF/BIOVIA-cosmoconf-datasheet.pdf .
- BIOVIA COSMOtherm, Release 2020; Dassault Systèmes. https://www.3ds.com/products/biovia/cosmo-rs/cosmotherm

