This is Part 1 of our 3-part blog series based on the ARC Advisory Group whitepaper, “Digital Transformation of the Pharmaceutical and Biotechnology Industries.” We’ve added a brief commentary about the 3DEXPERIENCE vision for how data analytics is driving the future of business transformation in these two key industries.
EXECUTIVE OVERVIEW
Compared to other consumer-focused industries, pharmaceutical and biotechnology have traditionally been slow to adopt emerging technologies. This is largely due to regulatory constraints, intellectual property issues, and a generally conservative culture. That said, most pharmaceutical and biotech companies are developing roadmaps for digital transformation and many have already started to adopt these newer digital technologies.
Digitalization allows sensors, machines, equipment, and people to communicate and collaborate, while providing real-time data to improve both plant processes and the products they create. Digitalization should enable new approaches for innovation and creativity as opposed to simply enhancing or supporting traditional approaches.
Digital transformation is beginning to disrupt the pharmaceutical, biotech, and medical device industries. Manufacturing companies are reinventing business processes and relationships and re-making legacy IT systems for better health care. This involves changes to business, engineering, supply chain, and manufacturing operations. The transformation involves modern software systems, data integrity, cloud, artificial intelligence, new healthcare sensors, robotics, connected things, and developing creative new ways to collaborate with and support patients.
For some companies, digital transformation, may just be a matter of going paperless (by digitizing paper-based data), connecting data silos, and/or implementing cloud-based solutions. But for most, digital transformation includes technologies such as cloud computing, advanced analytics in supply chain, cameras, video, augmented reality, and mobility.
While the Internet of Things (IoT) connects people, processes, data, and “things” over the internet; digital transformation includes integrating information technology (IT) and operational technology (OT) and data to enable better insights, optimize processes, and create efficiencies. The Industrial Internet of Things (IIoT) extends the IoT into industrial environments.
While we’re seeing some progress in adopting digital technologies for business and manufacturing processes in the pharmaceutical and biotech industries, manufacturers still struggle to exploit the full potential of digitalization. Often, cultural inertia—rather than technology—holds them back. ARC believes that digital technology will drive value for pharmaceutical and biotech manufacturing, which—ultimately—should drive widespread adoption.
Along with newer therapeutics such as genomics and personalized medicine, the industry is evolving and adapting for the digital transformation. Production processes will evolve to support higher volumes or adapt to personalized medicine with “batches of one.” Additionally, the industry is experiencing a cultural change that includes a newer generation of workers.
Major Challenges for the Pharmaceutical and Biotech Industry
In addition to operational problems, the pharmaceutical industry faces major challenges such as finding and gaining regulatory approvals for new drugs and then scaling up from laboratory to full production. The regulatory challenges demand new and improved track-and-trace solutions.
While as many as 70 percent of pharmaceutical companies are initiating pilots, ARC has not yet seen widespread expansions and scale up of digital transformation technologies across the enterprise, where the real value is to be found.
For some companies the initial strategy is to first improve cybersecurity and data integrity and move regulatory documents to the cloud. Others have begun advanced analytics initiatives and moved historian and other data to the cloud, edge, and new types of on-site devices. At least one major data historian supplier has a cloud offering that some pharma companies are using for real-time data. Other companies are implementing new enterprise architectures and platforms and scaling up digitalization, analytics, and visualization throughout the enterprise. Most of the companies that have not yet started on the digital transformation journey are now developing their initial plans and strategies.
The need for more efficient methods to produce breakthrough therapies and biosimilars, along with strategies to manufacture conventional and new drugs more economically and reliably sparks investments in more innovative methods for making drugs and biologics. The shift to personalized medicines and gene therapies will require the production of small batches. At the same time, pressure to reduce manufacturing costs and avoid shortages and recalls demands more reliable methods for ensuring the quality of large batches of conventional drugs.
Digital transformation enables manufacturing companies to allow patients to play a more active role in their own care. Some trends will impact manufacturing within the next few years, while others may take a little longer to adapt due to the transformation that will require changes in technologies, therapeutics, people, and processes.
Pharma & Biotech Industry Challenges, Causes and Potential Solutions
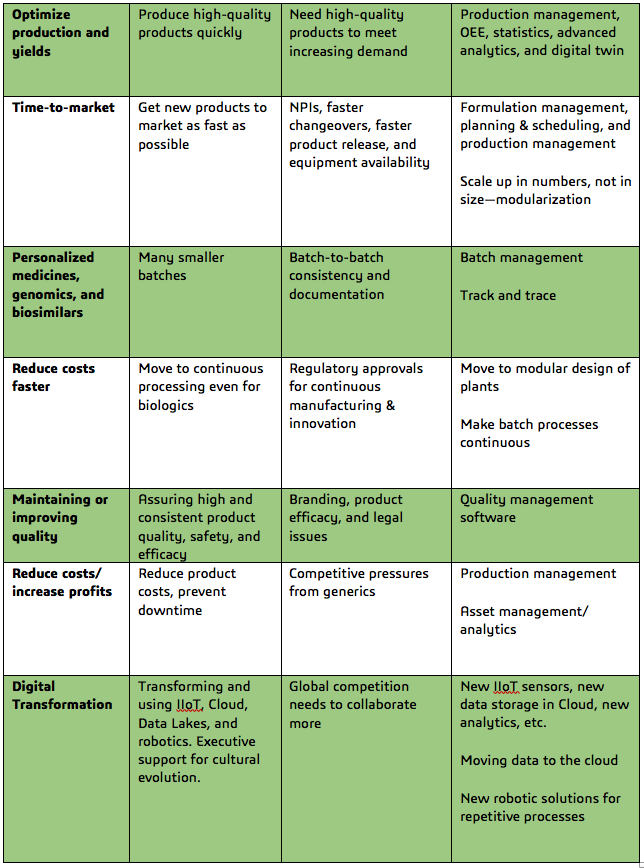
[Excerpt from the ARC Advisory Group whitepaper, “Digital Transformation of the Pharmaceutical and Biotechnology Industries”]
3DEXPERIENCE Commentary
In order to discover and produce tomorrow’s new breakthrough therapies, the pharma and biotech industries need to better leverage data intelligence to increase speed and agility.
Knowledge management is critical. According to current estimates, most scientific researchers waste 20 – 40% of their work time just searching for and accessing data. Overall, most companies make poor use of their existing knowledge and data: less than 10% of new data generated is ever analyzed. Likewise, some 40% of experiments are wasteful and unnecessarily repeated because the previously generated data and knowledge are not easily accessible.
Therefore, it is essential that pharma and biotech companies ensure on-demand access to data analytics across and between departments. By integrating and analyzing all data together, researchers can fully leverage existing scientific and experimental knowledge. Better intelligence leads to better therapies, faster.
For example, as the industry shifts to offer personalized medicines and gene therapies, production will move to small-batch manufacturing. This transformation will impact all production stages, requiring real-time data insights at every step for informed decision-making. Scientific discovery and research must leverage population-specific data to understand how new therapies affect different patients. In short, the ability to personalize medicine and better help a specific patient requires powerful data analytics.
Speeding time-to-market demands that manufacturers must eliminate redundant, repetitive work throughout the product lifecycle. Removing roadblocks is especially important in the research and development space, where experimental outcomes define the progress of a new therapeutic’s development.
Of course, breaking down legacy data silos and delivering analytics to researchers is easier said than done. Addressing these challenges requires organizations to better capture and share knowledge, enabling them to streamline operations end-to-end.
As more companies embrace digital transformation, we are seeing a huge increase in data generated today by sensors (Industrial Internet of Things) and robotics. The ever-increasing deluge of data makes it more and more difficult to find the right data at the right time and leverage it for critical insights. In addition, the increase in data complexity demands that data must be analyzed within its full context—including past project and real-time experimental results—in order to glean meaningful insights that can accelerate innovation. Many pharmaceutical companies today are consolidating information and applying analytics, but are unable to find the right data and ensure it is properly formatted.
Scientific Intelligence, powered by NETVIBES-EXALEAD and BIOVIA on the 3DEXPERIENCE platform, enables researchers to break down data silos and find the right data at the right time to accelerate innovation.
Learn more about Scientific Intelligence on the 3DEXPERIENCE platform.
In the next blog installment, we will look at how data analytics is driving digital transformation of the pharmaceutical and biotechnology industries.
Part 3: Critical Digital Transformation Initiatives in the Pharmaceutical & Biotechnology Industries
Download the ARC Whitepaper:

