“Light, seeking light, doth light of light beguile”, says a mocker in Shakespeare’s Love’s Labour’s Lost. This poetic musing captures a paradox inherent to light itself. Light is an everyday phenomenon, but its generation and absorption at the molecular level are still the subject of scientific research.
OLEDs: What Makes Them Shine?
Organic Light -Emitting Diodes (OLEDs) have transformed modern displays and lighting technologies. They power smartphones, televisions, laptops, VR/AR headsets, cars, medical devices, and ambient lighting. Their strengths are well known: vivid colors, thin form factors, flexibility, high contrast, fast response, and wide viewing angles.
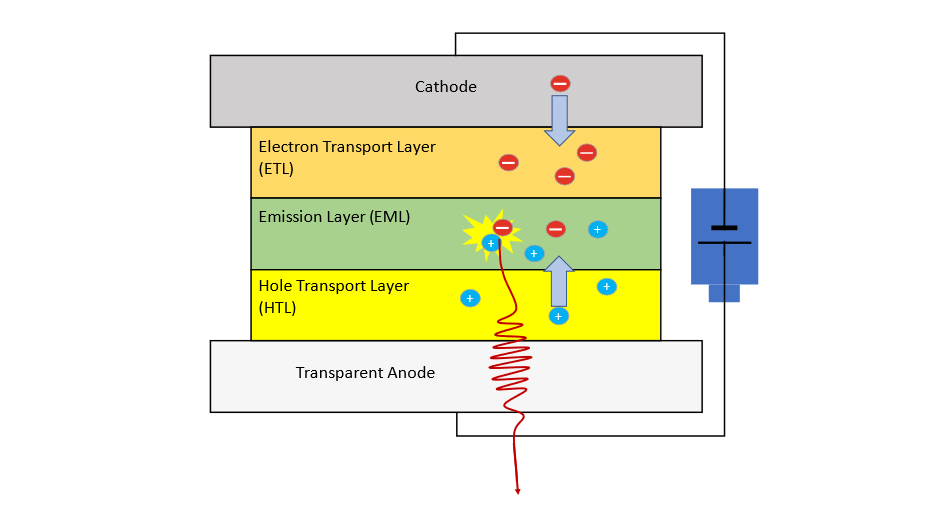
Figure 1: Scheme of an OLED stack. Each layer plays a critical role in efficiency and color.
An OLED is a stack composed of several layers of organic materials, each with a specific function. A sketch is shown in Figure 1. At the core is the emission layer, where light is generated. This layer is typically located between an electron transport layer (ETL) and a hole transport layer (HTL), all positioned between a negatively charged cathode and a positively charged anode. When a voltage is applied, electrons are injected from the cathode, while holes (positive charge carriers) are created at the anode. These charge carriers drift through their respective transport layers towards the emission layer, where they meet and recombine to form excited states called excitons. When these excitons relax to their ground state, they release energy in the form of light. The color of the emitted light is determined by the electronic structure of the emitter molecules. Therefore, precise chemical design is critical to OLED performance.
Despite their advantages, OLEDs still face a number of unresolved issues that researchers are actively working to solve. Major issues are lifetime and stability, as the materials are exposed to light and moisture, as well as constant electrical voltage and charge flow. This easily leads to decomposition reactions. Improving emitter and transport materials as well as optimizing device architectures are key research directions for novel stacks.
For the development of stable and efficient emitters, understanding how light is emitted is important. Different processes, like fluorescence and phosphorescence are possible. While phosphorescent OLEDs achieve nearly 100% internal quantum efficiency, fluorescent materials are still lacking this efficiency, resulting in higher power consumption in displays. The superior efficiency of phosphorescent OLEDs still comes at a price: Their reliance on rare heavy metals increases cost and sustainability concerns. Researchers are also exploring novel materials and concepts, such as thermally activated delayed fluorescence (TADF) and hybrid organic-inorganic emitters, to further expand the possibilities of producing light.
Other materials in an OLED stack, like electron/hole transport or blocking layers, can also be further optimized with an understanding of transport processes and adjusting conduction level.
Quantum Chemistry: Can We Understand Molecular Processes?
In the search for better materials, combining experiments with computational methods can have several advantages. Predicting the optical and electronic properties of helps to select the most promising candidates for further investigation. For instance, a large virtual chemical space can be screened for specific properties prior to synthesizing and measuring molecules. This can speed up the development process and reduce costs.
Computational methods uncover details of molecular and electronic structures that experiments alone can rarely capture. . Quantum chemistry, a branch of computational chemistry which applies quantum mechanical principles to chemical systems, is in particular an important method in OLED research. This is because other approaches, such as mechanistic or cheminformatics methods, lack information about electronic structure of molecules.
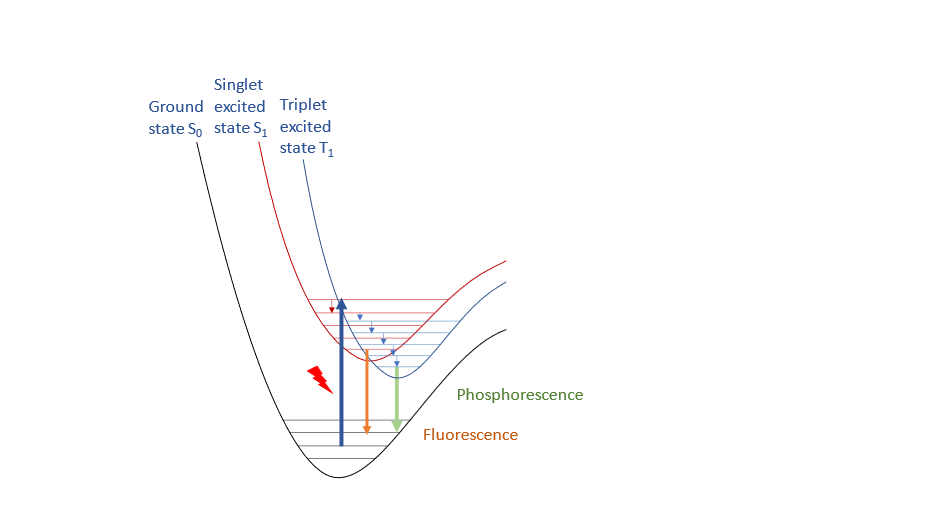
Figure 2: Scheme of fluorescence and phosphorescence processes.
Quantum chemistry is often viewed by chemistry students as something between difficult and useless or both. However, in OLED research in particular, it is worthwhile dealing with it, as it is the only way to understand fundamental mechanisms ab initio, i.e., largely without empirical assumptions.
Charge transport and light emission are at the heart of OLED functionality. Understanding both requires a correct mathematical description of electrons in molecules. The Schrödinger equation provides this description. Solving this equation with quantum chemical approaches yields internal energy levels and various properties can be derived. These energy levels determine, for example, the position of molecular orbitals that enable charge transport and the difference of electronic states involved in the excitation processes. For charge transport, the knowledge of the frontier orbitals of a molecule is of interest: the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbitals (LUMO), see Figure 3 for an example. Holes as positive charge carriers are created when an electron is removed from the HOMO, and the injection of electrons into the LUMO creates the negative charge carriers. Light is emitted when an electron relaxes from an excited state to its ground state and releases emission energy in the form of a photon, see Figure 2.
It is true that the details of quantum chemistry are difficult, but the application of quantum chemistry is also useful in real-life problems [1].
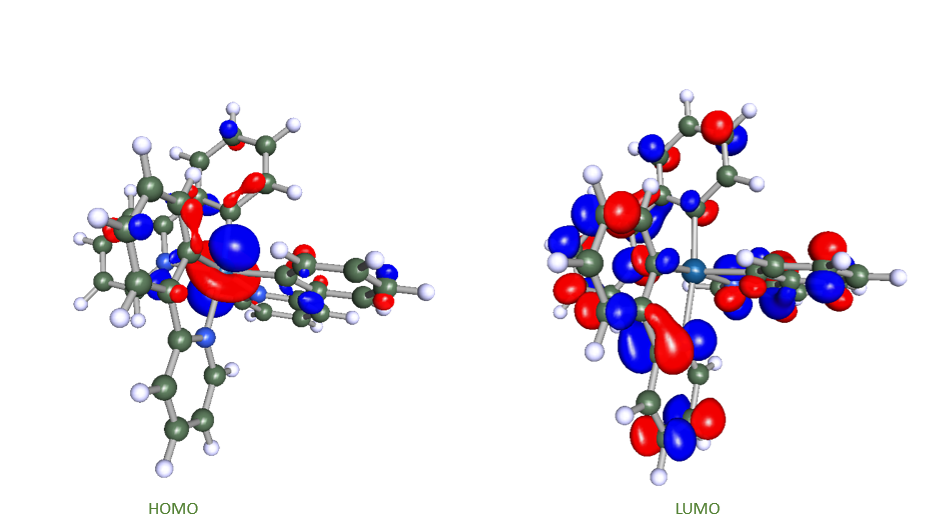
Figure 3: The frontier orbitals of Ir(ppy)3, a highly successful green phosphorescent emitter.
TURBOMOLE: How Can Computations Be Done In a Smart Way?
Quantum chemistry is not only daunting for beginners due to its mathematical formalism, but also for the user due to a zoo of methods and a plethora of shortcuts. Democratized solutions can help many users find their way through this jungle. There are various free and commercial programs available. These programs differ in terms of the selection of available methods, the efficiency with which they are implemented, how user-friendly they are, and how stable their programming is. In addition, they are often characterized by specialization or main areas of application. All of these factors should be considered when choosing a particular solution. BIOVIA offers the highly optimized software suite TURBOMOLE for quantum chemical computation [1].
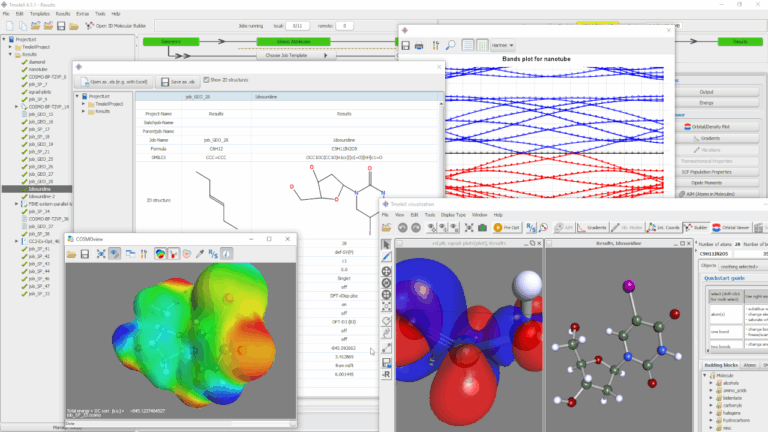
Figured 4: TURBOMOLE is the quantum chemical engine underlying various user interfaces (UIs).
Since its beginning as a Unix command line tool set, TURBOMOLE has become more and more democratized over the years. The graphical user-interface TmoleX is freely available for easy input creation, job processing, and result display [2]. With the implementation in Pipeline Pilot [3], TURBOMOLE can be integrated in scientific workflows. The 3DEXPERIENCE platform will make the value of quantum chemistry with TURBOMOLE obvious to non-experts. Figure 4 shows TURBOMOLE’s natural habitat.
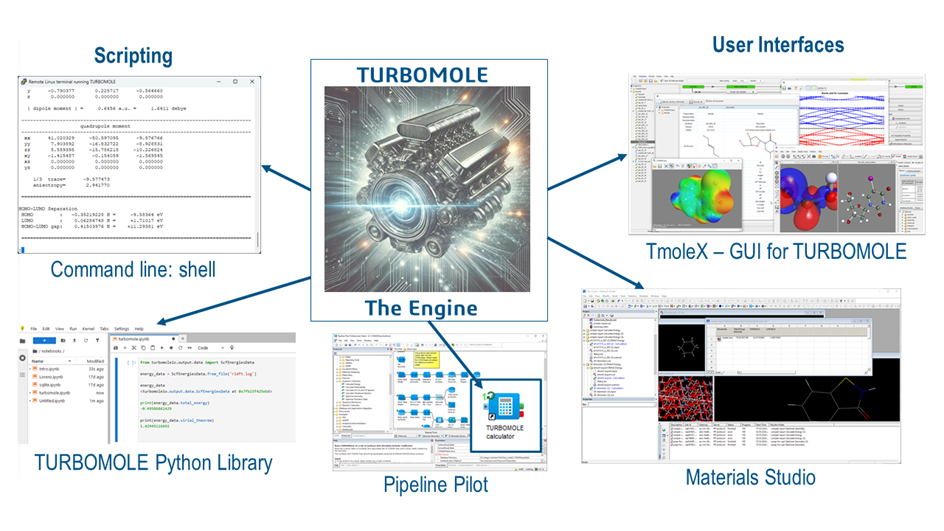
Figured 4: TURBOMOLE is the quantum chemical engine underlying various user interfaces (UIs).
Accompanying democratization, features have been added to the package over the years. According to the list of quantum chemistry software in Wikipedia, TURBOMOLE is currently the package with the most achieved feature characteristics, see Figure 5. Among the many features, lots that are important in OLED research are also included in the program package.
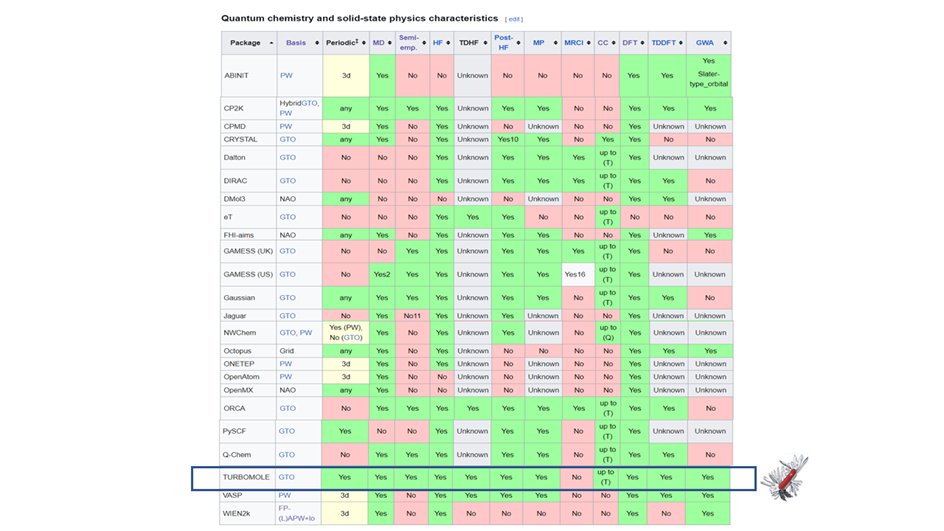
Figure 5: Source: adapted from January 2025.
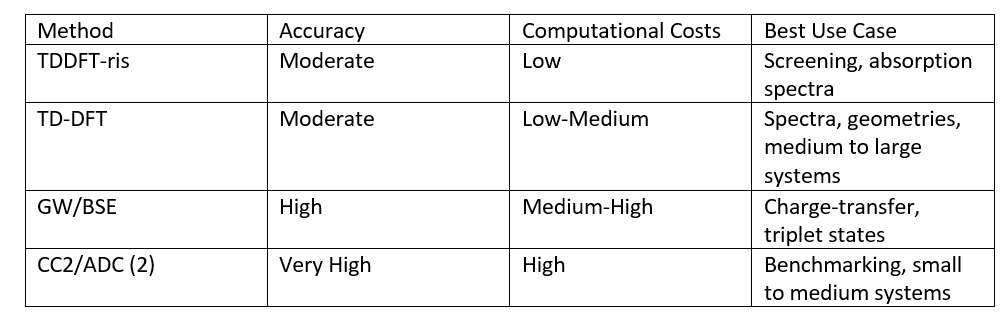
The workhorse for studying electronically excited states is time-dependent density functional theory (TD-DFT). With the semiempirical TDDFT-ris method, also a much faster approach for calculating TDDFT UV-Vis absorption is also available. For more accurate predictions, TURBOMOLE provides implementations of the GW approximation and Bethe–Salpeter equation (GW/BSE), the approximate coupled-cluster singles and doubles model CC2 and the algebraic diagrammatic construction through second-order [ADC(2)]. GW/BSE is post-processing of DFT calculations, while CC2 and ADC(2), in contrast, are non-DFT- based methods. Both significantly improve the accuracy for charge-transfer and triplet states. An overview of these methods and their typical applications is summarized in Table 1.
To capture the full complexity of photochemical processes, a combination of quantum-mechanical methods is required. A comprehensive description typically includes:
• Sound quantum mechanics for both ground and electronically excited states
• Accurate energies of the ground state as well as the singlet and triplet excited states
• Relaxation of excited-state geometries, since molecular structures can change dramatically upon excitation
• Vibrational frequencies of both ground and excited states, essential for spectra and dynamics
• Solvation effects, because the environment strongly influences excitation and relaxation pathways
• Spin-orbit coupling effects to enhance the efficiency by enabling the formation of triplet excitons into light-emitting states
For specialized studies, TURBOMOLE also provides advanced capabilities like quadratic response theory, vibrationally resolved spectroscopy, radiationless decay pathways, or nonadiabatic dynamics simulations. This means that the appropriate method for a certain photochemical problem can be selected depending on the task—from fast screening of molecular properties to highly accurate computations and advanced simulations of light-driven molecular processes.
Conclusion
In conclusion, OLED research sits at the intersection of molecular design, materials science, and quantum chemistry. Tools like BIOVIA TURBOMOLE— a powerful quantum-chemistry package from BIOVIA, DASSAULT SYSTEMES enable scientists to navigate this frontier swiftly and precisely, bringing brighter, more efficient displays from theory into our daily lives.
As computational power advances and computational methods become more accessible, quantum chemistry will play an increasingly critical and democratic role in accelerating materials research. With this foundation in place, the next act is poised to shine even more brilliantly.
Many thanks to Uwe Huniar for the enlightening conversations and for providing the images that helped bring the technical concepts in this article to life.
References
[1] Sree Ganesh Balasubramani, Guo P. Chen, Sonia Coriani, Michael Diedenhofen, Marius S. Frank, Yannick J. Franzke, Filipp Furche, Robin Grotjahn, Michael E. Harding, Christof Hättig, Arnim Hellweg, Benjamin Helmich-Paris, Christof Holzer, Uwe Huniar, Martin Kaupp, Alireza Marefat Khah, Sarah Karbalaei Khani, Thomas Müller, Fabian Mack, Brian D. Nguyen, Shane M. Parker, Eva Perlt, Dmitrij Rappoport, Kevin Reiter, Saswata Roy, Matthias Rückert, Gunnar Schmitz, Marek Sierka, Enrico Tapavicza, David P. Tew, Christoph van Wüllen, Vamsee K. Voora, Florian Weigend, Artur Wodyński, Jason M. Yu (2020). TURBOMOLE: Modular program suite for ab initio quantum-chemical and condensed-matter simulations. Journal of Chemical Physics; 152 (18), 184107.
[2] Claudia Steffen, Klaus Thomas, Uwe Huniar, Arnim Hellweg, Olvier Rubner, Alexander Schroer. (2010). TmoleX-a graphical user interface for TURBOMOLE. Journal of Computational Chemistry, 31(16), 2967-2970.
[3] Pipeline Pilot.
📩Want to find out the latest news about BIOVIA events, customer stories, blogs and more? Join the newsletter today!