Challenges
The rising concentration of CO2 in the atmosphere, primarily caused by human activities, is a significant driver of global warming. CO2 amplifies the greenhouse effect by absorbing infrared radiation, resulting in adverse climate changes that can increase the probability and severity of natural disasters like wildfires, heat waves, droughts, and storm surges.1 Therefore, scientists are exploring ways to directly capture CO2 from the atmosphere.2
Zeolites as Carbon Capture Solutions
Various CO2 capture technologies are available: chemical absorption, physical adsorption, membrane separation, and cryogenic separation. Among these options, adsorption technologies stand out due to their high CO2 uptake, low energy consumption, and non-toxic properties. Solid adsorbents such as activated carbon, zeolite, metal-organic frameworks (MOFs), organic-inorganic hybrid/composite adsorbents, porous polymers, carbon nanotubes, and silicon carbide are being employed for CO2 capture.3 Zeolites are particularly useful for CO2 capture because they belong to a versatile class of materials capable of selectively adsorbing CO2.4 They are widely used due to their affordability, availability, large surface area, porous texture, fast kinetics, and excellent chemical and thermal stability.
The word “zeolite” derives from the Greek words “zeô,” meaning to boil, and “lithos,” meaning rock (boiling rock). Indeed, zeolites are aluminosilicates composed of a framework of silicon and/or aluminum, as well as oxygen atoms. These oxygen atoms carry a negative charge, which is balanced by non-framework cations like sodium or calcium. Zeolites can trap water or other molecules within their pores.
As more than 200 types of zeolites are available5, including both natural and synthetic varieties, the goal of this project was to identify the optimal zeolite for adsorption. The ideal candidate would exhibit the highest adsorption capacity while selectively adsorbing CO2. While experimenting with each zeolite can be extremely time-consuming, molecular simulation not only offers a more efficient and cost-effective, but also a safer approach.
Leveraging BIOVIA Materials Studio for Zeolite Optimization
In this project, we utilized the Sorption module within BIOVIA Materials Studio 2023. This module enables the simulation of the absorption of a pure sorbate (in this case, the CO2 molecule) into a sorbent framework (the zeolite being tested). This framework comprises a three-dimensional periodic structure with pores of suitable size and shape to accommodate sorbate molecules. The Sorption module provides various functions for simulating the sorption process under different conditions (such as fixed pressure or fixed loading), calculating specific properties of the system (e.g., an adsorption isotherm), and identifying preferential sites for sorbate molecules within the framework.
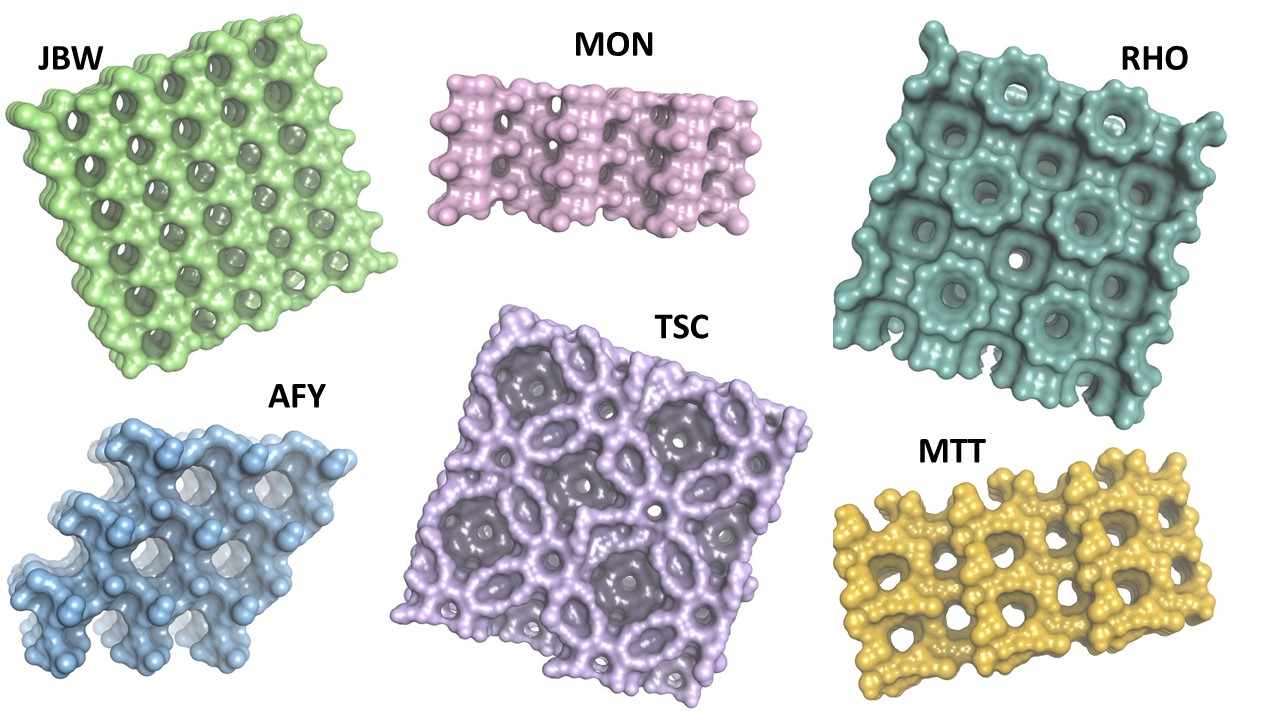
We initially computed adsorption isotherms for six zeolites. This involved conducting a series of fixed-pressure simulations at a constant temperature of 298 K in a single step. Each fixed-pressure simulation was executed over a specified number of steps within a pressure range set between 1 kPa and 1000 kPa. We used the same parameters as described in a recent paper from Okello et al.6
Throughout the simulation, CO2 molecules within the framework undergo random rotations and translations. Moreover, CO2 molecules are randomly introduced into or removed from the framework. The resulting configuration from each of these steps is either accepted or rejected based on the selection rules of the Monte Carlo method employed in the simulation.
In Figure 2a-f, we examine favorable binding sites within the lattice framework. In this visualization, we depict six different zeolites (see Figure 1), with dots symbolizing the CO2 adsorption sites, color-coded based on the density distribution of CO2. We choose to represent zeolites with diverse loading. The loading per cell and the percentage weight are provided for each simulated zeolite.
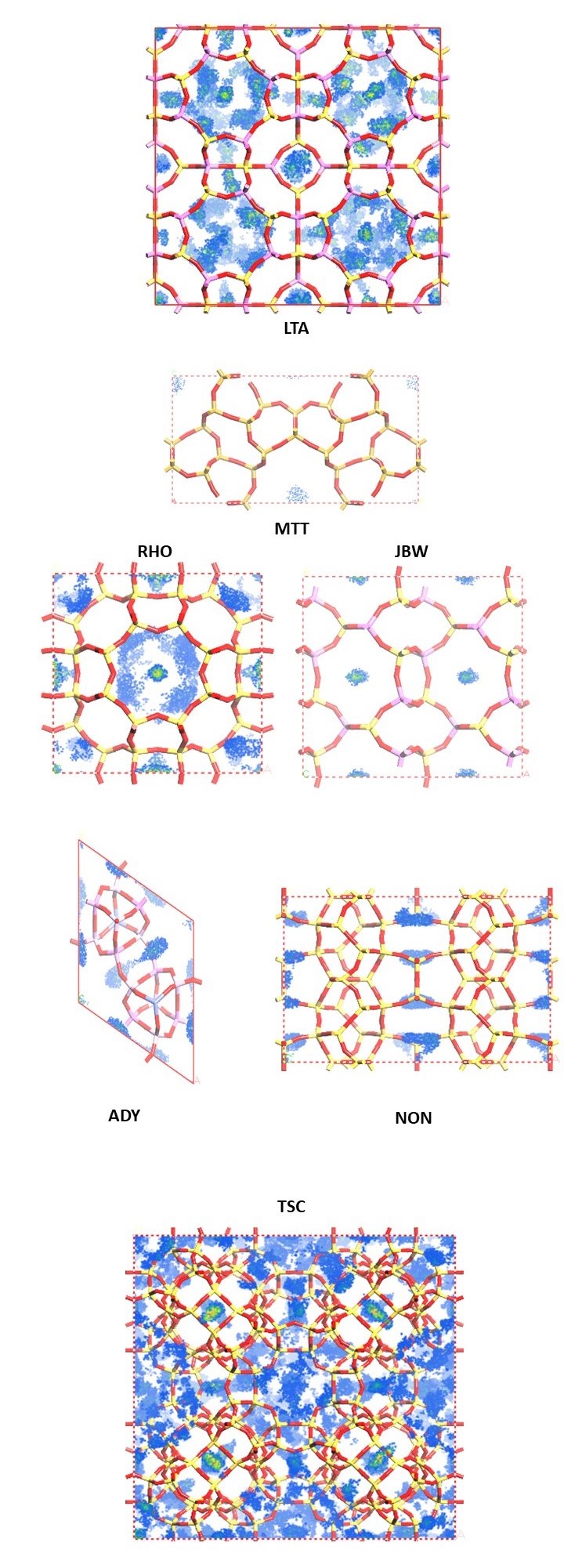
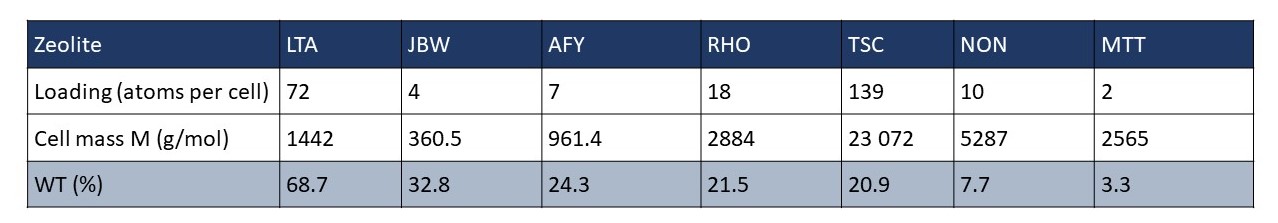
By analyzing Figure 2, we can distinguish the zeolite with superior adsorption capabilities from the others. However, another crucial factor that must be considered is the density of the zeolite, and consequently, the mass per cell. This is why we determine zeolite efficiency based on the weight percentage, calculated using the following formula:
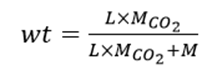
where L represents the loading, the CO2 molar mass, and the molar mass from the zeolite cell.
For this reason, LTA is the most effective adsorbing zeolite, boasting a weight percentage of 68.7%. On the other hand, one of the least promising zeolites was MTT, with a weight percentage of only 3.3%.
We also examined the lowest energy configuration for CO2 adsorption in LTA and it can be seen in the simulation video below that the size of the pores for LTA are optimal for CO2 adsorption.
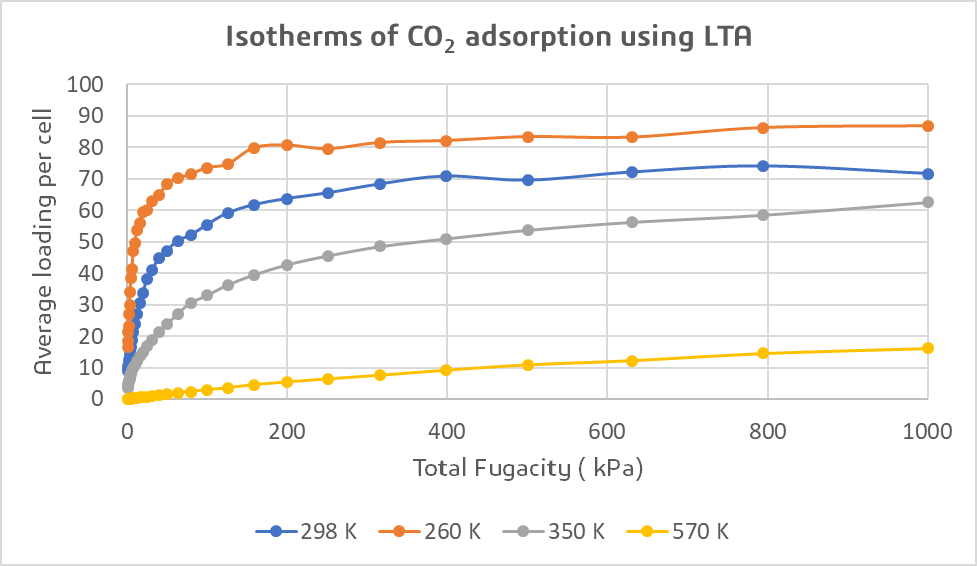
Temperature Effects on Zeolite Performance in CO2 Adsorption
Figure 3 clearly illustrates that as the temperature increases, the adsorption capacities decrease. The paper from Xiong et al.7 identifies two potential reasons that could explain this phenomenon. Firstly, adsorption is an exothermic process, so an increase in temperature shifts the reaction towards desorption. Additionally, as the temperature rises, the energy associated with the rotation and migration of the sorbate increases, resulting in reduced interactions between the sorbate and the zeolite.
Conclusion
Dealing with the surplus of atmospheric CO2 goes beyond environmental concerns. Its applications in industries and agriculture such as enhancing greenhouse plant growth are of great importance. Molecular simulations for zeolite optimization play a pivotal role. By refining zeolite properties, we facilitate swift CO2 adsorption and efficient desorption. Revealing the interactions between zeolites and CO2 is a catalyst for innovation, and zeolite-based solutions pave the way for harnessing the potential of CO2 for global industrial progress and environmental harmony.
References
[1] Mitchell, J.F., 1989. The “greenhouse” effect and climate change. Rev. Geophys. 27 (1), 115–139. doi: 10.1029/RG027i001p00115
[2] Bui, M., Adjiman, C.S., Bardow, A., Anthony, E.J., Boston, A., Brown, S., Fennell, P.S., Fuss, S., Galindo, A., Hackett, L.A., 2018. Carbon capture and storage (CCS): the way forward. Energy Environ. Sci. 11 (5), 1062–1176. doi: 10.1039/C7EE02342A.
[3] D’Alessandro D.M., Smit B., Long J.R., 2010. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed.;49:6058–6082.
[4] Boer, D.G, Langerak, J and. Pescarmona, P. P., 2023. Zeolites as Selective Adsorbents for CO2 Separation. ACS Applied Energy Materials. 6 (5), 2634-2656. doi:10.1021/acsaem.2c03605
[5] http://www.iza-structure.org/DatabaseHistory.htm
[6] Okello, F. O., Fidelis, T.T., Agumba, J., Manda, T., Ochilo, L., Mahmood, A., Pembere, A., 2023. Towards estimation and mechanism of CO2 adsorption on zeolite adsorbents using molecular simulations and machine learning. Materials Today Communications. 36, 106594. doi: 10.1016/j.mtcomm.2023.106594.
[7] Xiong, P., He, P., Qu, Y., Wang, L., Cao, Y., Xu, S., Chen, J., Ammar, M., Li, H., 2021. The adsorption properties of NaY zeolite for separation of ethylene glycol and 1,2-butanediol: Experiment and molecular modelling Green. Energy Environ. 6, 102–113. doi: 10.1016/j.gee.2019.12.006.

