Modeling and simulation tools are commonly used to speed up the drug development process – bringing an Active Pharmaceutical Ingredient (API) from the discovery stage to product development to manufacturing. During the development process (sometimes referred to as the ‘pre-formulation’ or ‘form selection’ step), pharmaceutical scientists must uncover the key physical properties of the New Chemical Entity (NCE): its chemical makeup, 3D (crystal) structure, stability, and solubility – this, to determine the final formulated product.
DASSAULT SYSTEMES BIOVIA offers software solutions for the various stages of development (such as ELN, Chemical registration systems, etc.). In particular, BIOVIA Modeling and Simulation tools such as Materials Studio®, can be used to perform crystal structure solutions or to look for potential polymorphic forms. At the early stage, researchers can predict crystal habits (morphology) and investigate the solvent effect on crystal growth. The compatibility or the solubility of the various components (or excipients) of the solid formulation can also be predicted using advanced quantum mechanical tools combined with the COSMO-RS method as implemented in BIOVIA Solvation Chemistry software.

The “compressed tablet” holds immense significance for global health and the economy as it is the most prevalent pharmaceutical dosage form. However, manufacturing issues frequently arise, particularly when introducing new drug formulations. These problems typically appear when production is escalated to a commercial scale, leading to elevated costs and significant disruptions.
Challenges in Tablet Manufacturing
A notable issue in tablet manufacturing is the adherence of powdered material to machinery, commonly known as “punch sticking”. This problem is particularly pronounced during the compression process and has several detrimental effects:
- Diminishes the visual appeal of the tablet, compromising its aesthetic quality.
- Impairs the uniformity of the Active Pharmaceutical Ingredient (API) dosage within the tablet.
- Reduces the cleanliness of the punch surface, further complicating the manufacturing process.
There are examples in the literature of molecular modeling used as a tool to address sticking: Hooper et al. investigated how the morphology of ibuprofen crystals affected their sticking propensity and linked it to the surface energy and surface polarity of the dominant faces of each habit [1]. More recently, Chaturvedi et al. attempted to predict drug-punch adhesive interactions by finding the interaction energy of individual ibuprofen, flurbiprofen, and ketoprofen molecules on an iron surface and comparing it to data from differential scanning calorimetry [2].
Various computational approaches (Figure 1) can be employed to study the interaction between the drug compounds and a punch steel surface.

Recently, our experts in Cambridge utilized experimental data [3] to test modeling approaches for predicting the affinity of drug compounds to adhere to steel surfaces.
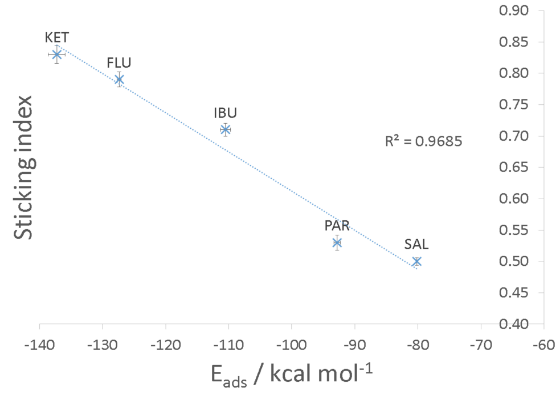
Specifically, a sophisticated Monte Carlo-based workflow was developed in Pipeline Pilot® to calculate the adhesion energies of these drugs (as molecules, as amorphous layers, or as crystal surfaces) on representative metal surfaces. In the first method, various conformers of the drugs were systematically generated and utilized in an in-house Simulated Annealing procedure to estimate the molecular adsorption energy (Fig 1.1). Subsequently, the obtained adsorption energy values were plotted against the experimentally reported Sticking Index. The results are shown in Figure 2, where the agreement between predicted adsorption energies and experimental sticking indices can be seen .
Conclusion
In summary, modeling and simulation in investigating the propensity for punch sticking during the tableting process offers significant advantages for the pharmaceutical industry. By considering factors such as excipient content, drug plasticity, and process parameters [4], researchers can comprehensively understand the underlying mechanisms and develop strategies to minimize sticking risks. This approach contributes to reducing late-stage risks, optimizing drug formulations, and improving the overall efficiency of the pharmaceutical manufacturing process.
[1] D. Hooper et al. Effects of crystal habit on the sticking propensity of ibuprofen—A case study. Int J Pharm 531 (2017) 266-275
[2] K. Chaturvedi et al. Modeling of Adhesion in Tablet Compression at the Molecular Level Using Thermal Analysis and Molecular Simulations. Mol Pharm 19 (2022) 26-34
[3] J.S. Saddik and R. H. Dave. Evaluation of powder rheology as a potential tool to predict tablet sticking. Powder Technology 386 (2021) 298-306
[4] S. Bennett and M. Meunier. Using simulation to investigate the issue of punch sticking during drug tablet manufacturing. To be published.

