
The choice of a proper solvent is crucial for many operations in the chemical and pharmaceutical industry. The range of applications in which the solvent plays a critical role extends from classic extraction, such as making your morning coffee, to more complex applications, such as selecting a solvent for chemical reactions. Here the solvent is used to dissolve the reacting species and at the same time, it influences the reaction rate and the product ratio.
Solvents can also have a significant influence in areas that do not come to mind more readily. An interesting example is plastic recycling, where selective dissolution can be used to separate the components of multilayer plastic films [1, 2], or the crystal morphology of an active pharmaceutical ingredient, which depends on the crystallization solvent [3].
When it comes to solvent selection, you will be spoiled for choice and In Silico prediction methods, like the Conductor-Like Screening Model for Real Solvents, COSMO-RS [4], which is implemented in the BIOVIA COSMOtherm software can be highly valuable. [5].
Due to its ability to use ready-made solvent molecule databases, and the fact that each calculation takes only a few seconds, the method is well-suited to solvent screening.
You can calculate a property of a compound in a large set of different solvents. The results can then be used to create a ranking of the best solvents and a subset of promising candidates can be used to perform measurements or further calculations. This procedure is not limited to pure solvents; any solvent mixture can be used.
An example of a COSMO-RS based solvent screening is given in figure 1. Here we use the BIOVIA Pipeline Pilot Solvation Chemistry Collection to realize an ionic liquid (IL) screening. The ILs form an interesting solvent group that has attracted a lot of interest during the last two decades. Their properties depend on the choice of the anion and the cation, which allows tailoring of the properties.
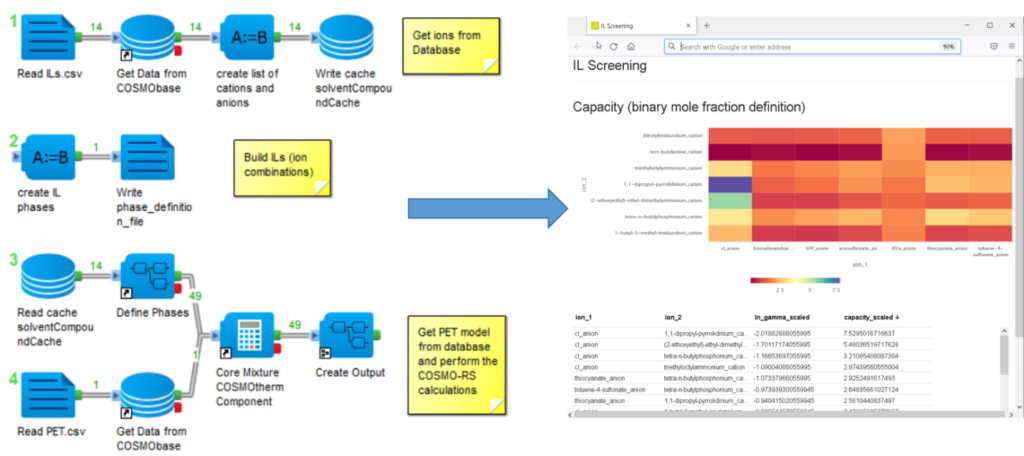
The Pipeline Pilot protocol used for the screening process in figure 1 consists of three steps. First, the ions are loaded from the database. The following step defines the IL phases, and they consist of a neutral mixture of a cation and an anion. In the final step, the properties of the solute in the IL phases are calculated.
Since the individual calculations do not take much time, we use all combinations of anions and cations as potential ILs and create a heat map and a sortable table as output. Ion parings not yet known as ILs can provide helpful information about important structural features. Alternatively, it is possible to use a predefined set of “real” ILs. In the example, we calculated the capacity of a Polyethylene terephthalate (PET) model in the different potential ILs. The capacity is the reciprocal of the activity coefficient and a good first approximation for the solubility of a substance in a liquid. Although we have not correctly described the solid state of the polymer in our PET model, the calculated values can still be used to rank the solvents, which gives us the most essential information: a set of promising solvents.
Virtual experiments, such as the COSMO-RS based solvent screening, save time and resources. They lead to a faster and more sustainable development process and are of great value in the search for green and benign substitutes for conventional fossil-based solvents.
- K. L. Sánchez-Rivera, P. Zhou, M. S. Kim, L. D. González Chávez, S. Grey, K. Nelson, S-C Wang, I. Hermans, V. M. Zavala, R. C. Van Lehn, G. W. Huber. Chem. Sus. Chem., 2021, 14, 4317. https://doi.org/10.1002/cssc.202101128
- M. Mohan, J. D. Keasling, B. A. Simmons and S. Singh. Green Chem., 2022, 24, 4140. https://doi.org/10.1039/d1gc03464b
- E. M. Soper, R. Y. Penchev, S. M. Todd, F. Eckert, M. Meunier, Journal of Crystal Growth, 2022, 591, 126712. https://doi.org/10.1016/j.jcrysgro.2022.126712
- A. Klamt. Wiley Interdiscip Rev. Comput. Mol. Sci. 2018, 8, 1338. https://doi.org/10.1002/wcms.1338
- M. González-Miquel, I. Díaz, Curr. Opin. in Green and Sustain. Chem., 2021, 29, 100469. https://doi.org/10.1016/j.cogsc.2021.100469
Learn more about:
