The regulatory landscape for Chemistry, Manufacturing, and Controls (CMC) dossier submissions is shifting decisively toward structured, data-driven submissions. As global standards like KASA, IDMP, and FHIR become the norm, traditional document-driven processes are proving inadequate. Regulatory bodies, including the FDA, are shifting toward structured data submissions to enhance consistency, transparency, and validation. This evolution demands a more dynamic, automated, and collaborative approach to dossier creation.
This shift from static documents to dynamic, data-driven workflows is a strategic necessity. By embracing automation and a data-first mindset, regulatory teams can achieve near real-time submissions, ensure end-to-end digital continuity, and maintain unparalleled consistency across all documentation. This post explores how a data-centric approach, powered by Deterministic and Generative AI can streamline dossier creation and set a new standard for regulatory excellence.
The Technology Backbone of Regulatory Transformation
Several interconnected technologies form the foundation of an automated CMC dossier solution. When integrated on a single platform they create a powerful ecosystem that turns raw data into submission-ready content with integrity and speed.
Ontology Management and Semantic Graphs
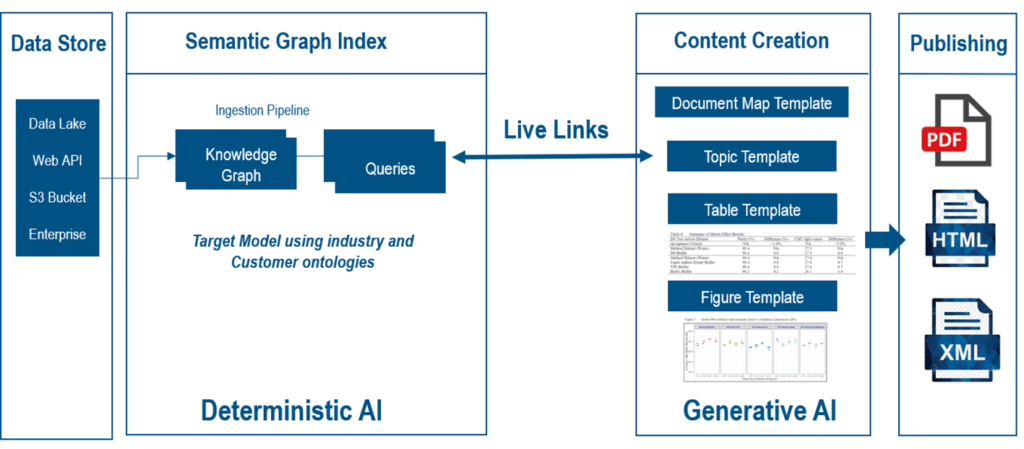
At the core of this transformation is Ontology Management. An ontology is a formal regulatory data model that defines how CMC data elements relate and validate each other. For CMC, this means creating a structured framework that aligns with regulatory requirements. It establishes a common language for all data, from lab results to manufacturing specifications. This semantic backbone ensures consistency and improves data governance, with the “Data Steward” role becoming accountable for maintaining regulatory consistency across the entire data lifecycle.
Building on this, Semantic Knowledge Graphs connect diverse datasets in a deeply contextual way. Instead of storing data in isolated silos, a knowledge graph creates a web of interconnected information. This allows for more sophisticated queries and provides a holistic view of the data, revealing insights that would be difficult to find with traditional methods.
Structured Authoring and Deterministic AI
Structured authoring serves as the foundation for transforming knowledge graph data into well-defined, pre-templated tables, charts, and other essential data elements. Because the knowledge graph is continuously refreshed in near real time, the content within the structured authoring environment can likewise be updated until a specific section, paragraph, or chapter is formally frozen and locked from further edits. Structured authoring effectively provides the front-end interface through which authors can observe, curate, and narrate the text associated with these tables and charts, all of which is driven by Deterministic AI. This ensures there is no distortion in the data pathway from the source to the structured authoring environment—the data presented as tables and charts remains fully traceable and reliable.
Once the appropriate tables and charts are created, you can, if you wish, use a Large Language Model (LLM) to generate the corresponding narrative. This must be a collaborative effort between the LLM and the author, who remains responsible for continually assessing, curating, and verifying any content generated by Generative AI. Of course, LLMs should not be trusted without human oversight in regulated CMC contexts. But the combination of a Deterministic data flow to create the tables and an LLM to generate the narrative gives us powerful accelerators toward automating CMC end to end.
The Shift to FHIR
The healthcare and life sciences industry is rapidly embracing the Fast Healthcare Interoperability Resources (FHIR) standard for data exchange. Regulatory bodies such as the EMA and FDA are increasingly mandating or encouraging its use for dossier submissions, and legacy formats like PDF and SPL are becoming obsolete. By integrating deterministic mapping from internal data ontologies to FHIR standards, organizations ensure their regulatory operations are not only compliant with current mandates but also ready for future requirements such as FHIR R6. Automated platforms capable of generating FHIR-native bundles directly from live source data reduces rework as regulatory data standards evolve, eliminating manual conversion tasks and future-proofing the dossier submission process.
Tangible Value of a Data-Driven Approach
Adopting an automated, data-centric model for CMC dossier creation with a unified platform delivers measurable value across the organization. Industry benchmarks consistently show cost savings, reduction in the time needed to create stability and batch analysis sections, and decrease in overall CMC content authoring time. These efficiencies accelerate the entire submission timeline, allowing organizations to bring products to market faster.
Reduce Time and Costs
Automating manual processes significantly reduces non-value-added tasks. Teams can eliminate repetitive data verification, reduce review cycles, and shorten response times to health authorities. This leads to right-first-time submissions and fewer post-submission issues, accelerating the path to market.
Enhance Quality and Compliance
Automation minimizes errors caused by manual data entry and transfer. It ensures the consistency and completeness of data and documents while providing end-to-end traceability. This “data integrity by design” approach helps organizations keep up with evolving agency requirements, including new data formats like FHIR.
Improve Collaboration
A unified platform removes the complexity of working across multiple, disconnected systems. Teams can collaborate simultaneously on submissions, share knowledge through structured content, and formalize internal best practices. This streamlined environment also makes it easier to integrate new partners and suppliers into the workflow, fostering greater efficiency.
The 3DEXPERIENCE Platform: A Unified Solution
The 3DEXPERIENCE platform is an integrated, end-to-end environment for data-driven CMC dossier creation. It is a semantically aware environment where ontologies inform the data model, delivering a comprehensive automation solution. By moving from a document-centric to a data-driven model, organizations can automatically generate new CMC documents from a wide variety of sources.
The platform’s data science applications allow teams to align, aggregate, and add semantics to incoming data. This enables the automated creation of data tables through queries or events. Data from sources like stability, specification, and batch analysis can be harmonized into a structured, well-informed view aligned with the regulatory model.
Key capabilities include:
- Parameterized templates for automated content creation
- Collaborative real-time authoring environment for technical documentation
- Live data links from the semantic graph index
- Full control of published output and content reuse at section, table, and image levels
- Lifecycle management of individual content sections
- Graphical revision history for complete traceability of all changes
This collaborative environment allows multiple authors to work simultaneously while maintaining control over content maturity. The system combines live data links with human narrative, generating submission content with exceptional automation and integrity.
The Future of Regulatory Submissions
The automation of CMC dossier creation is no longer a distant vision; it is a present-day reality with proven benefits. By consolidating data, leveraging AI, and adopting a structured, platform-based approach, life sciences companies can achieve significant gains in efficiency, quality, and compliance. The regulatory landscape will continue to evolve, and the technologies to meet these new demands are mature and available. By taking the first step toward a data-centric model, your organization can begin the journey toward a more agile, compliant, and innovative regulatory future.
Explore how end-to-end automation can remove manual CMC rework while strengthening compliance in the CMC dossier creation process.
📩Want to find out the latest news about BIOVIA events, customer stories, blogs and more? Join the newsletter today!

