This post originally appeared in the Arieh Halpern.
Do no harm. Medicine’s first principle is absolutely put to the test during the new product development and innovation cycle for “high-risk” medical devices such as implants. While research and product development teams want to investigate and prove out every concept and potential outcome, they need to reduce the chance of unanticipated negative effects. What does that mean for animal studies and human clinical trials?
Computational Modeling and Simulation (CM&S) lets you create and simulate human biologic test environments and conditions to complement the validation of safety and effectiveness by nonphysical means—not to mention accelerate development and lower cost. This not only reduces the risk of physical testing and speeds getting products to patients—CM&S can go beyond what is possible to explore within traditional end-product tests. In its virtual environments, you can define and model any patient condition to gather data to improve upon product design, performance, and patient safety.
Testing on animals and humans is limited by the practical consideration of trying to replicate the conditions within an animal or human body. Often, even when these conditions are replicable, testing raises ethical considerations that must be adhered to in order to not expose the patient to harm.
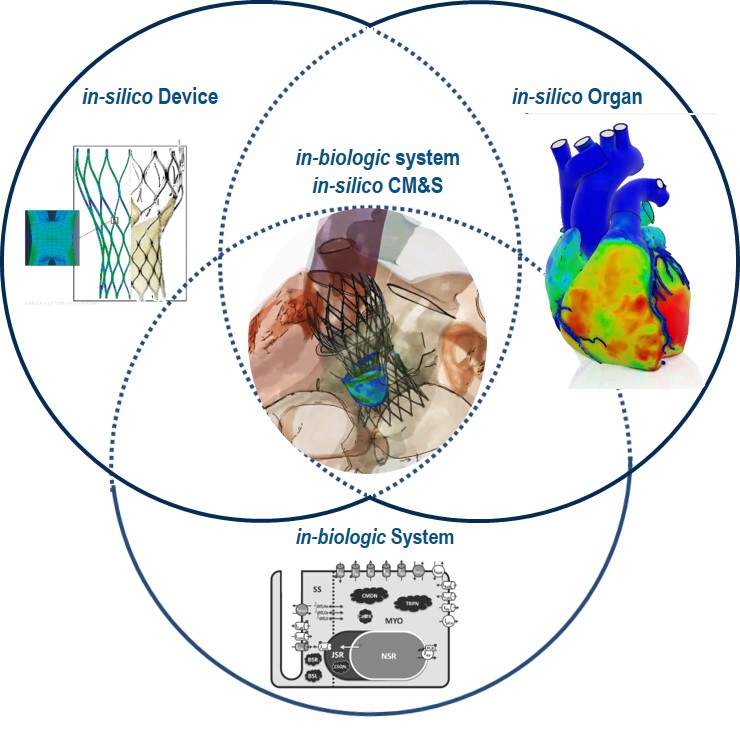
The state of the science is now “in-biologic system in-silico CM&S” (see Figure 1)—a “virtually real” capability that lets life science companies create biological models of how devices will operate within a living patient. For example, modeling an artificial heart valve—within a multi-dimensional, physiologically accurate
Since an implant ought to be “for life,” CM&S can also be used to simulate performance over long periods of time and under various conditions, and to model long-term drug interactions on the implanted materials. CM&S also addresses the increasing complexity of medical device systems. Nowadays, these can contain interrelated mechanical, chemical, biological, electrical, electronic, software, fluid, and other subsystems—each of which might include multiple assemblies of parts, and the whole of which must operate effectively and safely within the human organism. CM&S has the compute power and scope to handle these massive system engineering simulations and computations, running on a digital, collaborative innovation platform so enterprise and ecosystem stakeholders can view, explore, and utilize the virtual prototype model for different purposes and perspectives. The U.S. Food and Drug Administration has taken note—digital evidence is already being used at the FDA Center for Devices and Radiological Health (CDRH) to support regulatory submissions. Tina Morrison, PhD, Deputy Director in the Division of Applied Mechanics at the CDRH, was the lead author of a paper published online last September in Frontiers in Medicine, “Advancing Regulatory Science With Computational Modeling for Medical Devices at the FDA’s Office of Science and Engineering Laboratories.” Excerpts from the abstract cut to the chase: “… Computational modeling has become an increasingly powerful tool for evaluating medical devices, complementing bench, animal and clinical methods… computational modeling methods are increasingly being used within software platforms, serving as clinical decision support tools… because of its reach and huge potential, computational modeling has been identified as a priority by CDRH, and indeed by FDA’s leadership.” Note: Additional authors of this post include Darcy Sheerin, Steven Levine, PhD and Stuart Wright, PhD, Dassault Systèmes